A very small cross-sectional study (= there's one time point) from Russia has just been published (well, in April, but it just showed up on PubMed now). The study included 36 vegans, 38 vegetarians (lacto-ovo, I'm assuming), and 29 omnivores, all apparently healthy. The age range was large: 18 to 77 years.

The study found:
- Lumbar spine osteopenia: "Osteopenia in the lumbar spine was diagnosed in 27.8% of vegans, 39.5% of vegetarians, and 31.0% of omnivores."
- Femoral neck osteopenia: "In the femoral neck, [bone mineral density] corresponding to osteopenia [i.e., low bone mineral density] was detected in 19.4[% of vegans], 26.3[% of vegetarians], and 17.2% of [omnivores] [..]."
- Lumbar spine osteoporosis: "18.4% of vegetarians and 6.9% of omnivores had [bone mineral density] corresponding to osteoporosis [i.e., very low bone mineral density] in the lumbar spine." None of the vegans had osteoporosis in the lumbar spine.
- Femoral neck osteoporosis: None of the participants had this.
Interesingly: "Excluding people who had took vitamin D supplements regularly did not drastically change the results of the study." 26 of the 36 vegans (i.e., >72%) did not seem to take a vitamin D supplement regularly.
Note: The relatively good bone health of the vegan participants in this study could have been influenced by their relativey young age, even though the age range was mostly different compared to the vegetarians, not compared to the omnivores. The authors do not say where the participants were from (apart from that they were in Russia). Presumably, the participants were all residents of Moscow (my guess).
The authors' conclusion:
"The findings suggest that [bone mineral density] in vegans and vegetarians in Russia does not differ from that in omnivores. However, further larger studies are required."
Reference:
- A V Galchenko, E I Sidorova, K M Gapparova, A A Sherstneva, V A Revyakina: [Bone mineral density in vegetarians and vegans], Vopr Pitan. 2023;92(3):69-78. doi: 10.33029/0042-8833-2023-92-3-69-78. Epub 2023 Apr 17. [Article in Russian]
Full text:
The full text in Russian is available here: Показатели минеральной плотности костной ткани у вегетарианцев и веганов. ...
... But for those of you who don't like Russian, here's an English translation of the full text (see below).
Original abstract (originally in English, i.e., as written by the authors of the paper):
"
The number of vegetarians and vegans is increasing each year. In this regard, studies of the quality of diets that exclude slaughter foods, as well as their impact on human health, are becoming more and more relevant. The main purpose of the study was to assess the bone mineral density (BMD) in Russian vegetarians and vegans, as well as in omnivores.
Material and methods. Design - cross-sectional study. On an outpatient basis, we examined 103 conditionally healthy people aged 18 to 77 years with different diets: 36 vegans, 38 vegetarians and 29 omnivores. X-ray two energy absorptiometry was used to assess BMD. The density of the lumbar vertebrae (LI-LIV) and femoral neck was measured.
Results. Osteopenia in the lumbar spine was diagnosed in 27.8% of vegans, 39.5% of vegetarians, and 31.0% of omnivores. In the femoral neck, BMD corresponding to osteopenia was detected in 19.4, 26.3, and 17.2% of cases, respectively. 18.4% of vegetarians and 6.9% of omnivores had BMD corresponding to osteoporosis in the lumbar spine. Osteoporosis was not diagnosed in the femoral neck. No significant differences were observed after exclusion of people over 50 years of age. This was probably due primarily to the fact that the largest number of peri and postmenopausal women were in the vegetarian group. Excluding people who had took vitamin D supplements regularly did not drastically change the results of the study. When taking into account both exclusion criteria, no significant differences were observed.
Conclusion. The findings suggest that BMD in vegans and vegetarians in Russia does not differ from that in omnivores. However, further larger studies are required.
"
Osteoporosis is a systemic skeletal disease characterized by low bone mineral density (BMD) and impaired microarchitecture, which increase the risk of fractures [8, 9]. Depending on age and sex, it can be distinguised between idiopathic juvenile osteoporosis, idiopathic adult osteoporosis, postmenopausal osteoporosis, and senile osteoporosis. Osteoporosis can also develop in associatioon with a lack of physical activity, with endocrine, hematological, oncological, nephrological, gastroenterological, neurological, rheumatological diseases, or as due to iatrogenic effects, etc. [10].
Despite ample evidence of the beneficial effects of limiting the consumption of animal products and refined plant foods [11-13], the impact of dietary habits, including vegetarianism, on BMD is not fully understood. In this regard, BMD studies with vegans and vegetarians are very relevant today.
The aim of the [present] work was to study BMD in vegetarians, vegans, and people with a mixed diet [omnivores].
We did not find any such studies [on the topic of BMD in vegetarians/vegans] in Russia. In addition to the climatic and geographical features of the European territory of Russia, a huge contribution to the health of the population is also made by the features of economic development, the material well-being of the population, the national mentality, the availability of food products, and culinary preferences [this sound a little overenthusiastic]. All of the above predetermined the need to conduct a study of the state of bone tissue [bone health] in vegetarians and vegans in Russia.
It is known that physical activity interferes [which is a good thing] with bone resorption [27]. Vegetarians, especially women who are more active, may have lower bone resorption compared to mixed dieters [28, 29]. In a study by R. Wakolbinger-Habel et al. [30] found no differences in bone microarchitecture between vegans with regular strength training and omnivores, although quantitative indicators of BMD in non-exercising vegans were lower when compared with a similar group of omnivores.
The present study found no reduction in BMD in vegans compared to omnivores, other things being equal. The differences were mainly due to the large number of older persons among the surveyed vegetarians compared to the other [two] groups. At the same time, the fact of regular intake of vitamin D did not have the expected effect on the results of the study.
2. Chai Z.F., Gan W.Y., Chin Y.S., Ching Y.K., Appukutty M. Factors associated with anemia among female adult vegetarians in Malaysia // Nutr. Res. Pract. 2019. Vol. 13, N 1. P. 23. DOI: https://doi.org/10.4162/nrp.2019.13.1.23
3. Исследовательское агентство Zoom Market. URL: https://www.mazm.ru/article/a-2122.php (дата обращения: 28.03.2019).
4. Elorinne A., Alfthan G., Erlund I. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians // PLoS One. 2016. Vol. 11, N 2. Article ID e0148235. DOI: https://doi.org/10.1371/journal.pone.0148235
5. Kahleova H., Levin S., Barnard N. Cardio-metabolic benefits of plant-based diets // Nutrients. 2017. Vol. 9, N 8. P. 848. DOI: https://doi.org/10.3390/nu9080848
6. Zhubi-Bakija F., Bajraktari G., Bytyçi I., Mikhailidis D.P., Henein M.Y., Latkovskis G. et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the International Lipid Expert Panel (ILEP) // Am. J. Clin. Nutr. 2020. Vol. 40, N 1. P. 255-276. DOI: https://doi.org/10.1016/j.clnu.2020.05.017
7. Galchenko A., Gapparova K., Sidorova E. The influence of vegetarian and vegan diets on the state of bone mineral density in humans // Crit. Rev. Food. Sci. Nutr. 2023. Vol. 63, N 7. Р. 845-861. DOI: https://doi.org/10.1080/10408398.2021.1996330
8. Lane J.M., Russell L., Khan S.N. Osteoporosis // Clin. Orthop. Relat. Res. 2000. Vol. 372. P. 139-150. DOI: https://doi.org/10.1097/00003086-200003000-00016
9. Karaguzel G., Holick M.F. Diagnosis and treatment of osteopenia // Rev. Endocr. Metab. Disord. 2010. Vol. 11, N 4. P. 237-251. DOI: https://doi.org/10.1007/s11154-010
10. Мельниченко Г.А., Белая Ж.Е., Рожинская Л.Я., Торопцова Н.В., Алексеева Л.И., Бирюкова Е.В. и др. Федеральные клинические рекомендации по диагностике, лечению и профилактике остеопороза // Проблемы эндокринологии. 2017. Т. 63, № 6. С. 392-426. DOI: https://doi.org/10.14341/probl2017636392-426
11. Hu J., Li Y., Wang Z., Li X., Hou T., Ning Z. et al. Association of plant-based dietary patterns with the risk of osteoporosis in community-dwelling adults over 60 years: a cross-sectional study // Osteoporos. Int. 2023. Vol. 34, N 5. Р. 915-923. DOI: https://doi.org/10.1007/s00198-023-06700-2
12. Ghadiri M., Cheshmazar E., Shateri Z., Gerami S., Nouri M., Gargari B.P. Healthy plant-based diet index as a determinant of bone mineral density in osteoporotic postmenopausal women: a case-control study // Front. Nutr. 2023. Vol. 9. Article ID 1083685. DOI: https://doi.org/10.3389/fnut.2022.1083685
13. Zheng Y., Wang J., Wang Y., Xu K., Chen X. The Hidden dangers of plant-based diets affecting bone health: a cross-sectional study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005-2018 // Nutrients. 2023. Vol. 15, N 7. Р. 1794. DOI: https://doi.org/10.3390/nu15071794
14. Galchenko A.V., Ranjit R. Calcium status among vegetarians and vegans // Российской научно-практической конференции с международным участием "Фундаментальные основы технологического развития сельского хозяйства" : сборник трудов. Оренбург: Федеральное государственное бюджетное научное учреждение "Федеральный научный центр биологических систем и агротехнологий Российской академии наук", 2019. С. 214-217.
15. Galchenko A.V., Ranjit R. Vitamin D and its status in vegetarians and vegans // Вопросы биологической, медицинской и фармацевтической химии. 2021. Т. 24, № 11. С. 20-27. DOI: https://doi.org/10.29296/25877313-2021-11-04
16. Шкарабуров А.С., Колпинский Г.И., Захаров И.С., Шкарабуров С.П., Мозес В.Г. Использование лучевых методов в диагностике постменопаузального остеопороза // Фундаментальная и клиническая медицина. 2017. Т. 2, № 2. С. 70-76. DOI: https://doi.org/10.23946/2500-0764-2017-2-2-70-76
17. Oral A., Esmaeilzadeh S., Yalıman A., Sindel D., Kürsüz Köseoğlu P., Aydın T. The ability of calcaneal and multisite quantitative ultrasound variables in the identification of osteoporosis in women and men // Turk. J. Phys. Med. Rehabil. 2019. Vol. 65, N 3. P. 203-215. DOI: https://doi.org/10.5606/tftrd.2019.1894
18. Ho-Pham L.T., Vu B.Q., Lai T.Q., Nguyen N.D., Nguyen T.V. Vegetarianism, bone loss, fracture and vitamin D: a longitudinal study in Asian vegans and non-vegans // Eur. J. Clin. Nutr. 2012. Vol. 66, N 1. P. 75-82. DOI: https://doi.org/10.1038/ejcn.2011.131
19. Craig W.J. Health effects of vegan diets // Am. J. Clin. Nutr. 2009. Vol. 89, N 5. P. 1627S-1633S. DOI: https://doi.org/10.3945/ajcn.2009.26736N
20. Welch A., Bingham S., Camus J., Dalzell N., Reeve J., Day N., Khaw K.T. Calcaneum broadband ultrasound attenuation relates to vegetarian and omnivorous diets differently in men and women: an observation from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study // Osteoporos. Int. 2005. Vol. 16, N 6. P. 590-596. DOI: https://doi.org/10.1007/s00198-004-1721-3
21. Ho-Pham L.T., Nguyen N.D., Nguyen T.V. Effect of vegetarian diets on bone mineral density: a Bayesian meta-analysis // Am. J. Clin. Nutr. 2009. Vol. 90, N 4. P. 943-950. DOI: https://doi.org/10.3945/ajcn.2009.27521
22. Xie L., Wang B., Cui X., Tang Q., Cai W., Shen X. Young adult vegetarians in Shanghai have comparable bone health to omnivores despite lower serum 25(OH) vitamin D in vegans: a cross-sectional study // Asia Pac. J. Clin. Nutr. 2019. Vol. 28, N 2. P. 383-288. DOI: https://doi.org/10.6133/apjcn.201906_28(2).0021
23. Chuang T.L., Koo M., Chuang M.H., Lin C.H., Huang C.H., Wang Y.F. Changes in bone mineral density and trabecular bone score over time between vegetarian and non-vegetarian middle-aged and older women: a three-year retrospective medical record review // Int. J. Environ. Res. Public Health. 2022. Vol. 19, N 4. Р. 2445. DOI: https://doi.org/10.3390/ijerph19042445
24. Movassagh E.Z., Baxter-Jones A.D.G., Kontulainen S., Whiting S., Szafron M., Vatanparast H. Vegetarian-style dietary pattern during adolescence has long-term positive impact on bone from adolescence to young adulthood: a longitudinal study // Nutr. J. 2018. Vol. 17, N 1. P. 36. DOI: https://doi.org/10.1186/s12937-018-0324-3
25. Menzel J., Abraham K., Stangl G.I., Ueland P.M., Obeid R., Schulze M.B. et al. Vegan diet and bone health - results from the cross-sectional RBVD study // Nutrients. 2021. Vol. 13, N 2. Р. 685. DOI: https://doi.org/10.3390/nu13020685
26. Penczynski K.J., Remer T., Menzel J., Abraham K., Weikert C. Urinary potential renal acid load (uPRAL) among vegans versus omnivores and its association with bone health in the cross-sectional risks and benefits of a vegan diet study // Nutrients. 2022. Vol. 14, N 21. Р. 4468. DOI: https://doi.org/10.3390/nu14214468
27. Aguado E., Pascaretti-Grizon F., Goyenvalle E., Audran M., Chappard D. Bone mass and bone quality are altered by hypoactivity in the chicken // PLoS One. 2015. Vol. 10, N 1. Article ID e0116763. DOI: https://doi.org/10.1371/journal.pone.0116763
28. Bedford J.L., Barr S.I. Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more "health conscious" // Int. J. Behav. Nutr. Phys. Act. 2005. Vol. 2, N 1. P. 4. DOI: https://doi.org/10.1186/1479-5868-2-4
29. Tong T.Y.N., Appleby P.N., Armstrong M.E.G., Fensom G.K., Knuppel A., Papier K. et al. Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study // BMC Med. 2020. Vol. 18, N 1. P. 353. DOI: https://doi.org/10.1186/s12916-020-01815-3
30. Wakolbinger-Habel R., Reinweber M., König J., Pokan R., König D., Pietschmann P. et al. Self-reported resistance training is associated with better HR-pQCT-derived bone microarchitecture in vegan people // J. Clin. Endocrinol. Metab. 2022. Vol. 107, N 10. Р. 2900-2911. DOI: https://doi.org/10.1210/clinem/dgac445
31. Гальченко А.В., Морозова Л.Д., Залетова Т.С. Оценка потребности в белке и аминокислотах, исходя из биосинтетических потребностей и показателей азотистого баланса // Вопросы диетологии. 2017. Т. 7, № 2. С. 64-68. DOI: https://doi.org/10.20953/2224-5448-2017-2-64-68
32. Гальченко А.В. Влияние факторов образа жизни на метаболизм костной ткани и риск развития остеопороза // Профилактическая медицина. 2022. Т. 25, № 6. С. 96-107. DOI: https://doi.org/10.17116/profmed20222506196
33. Falchetti A., Cavati G., Valenti R., Mingiano C., Cosso R., Gennari L. et al. The effects of vegetarian diets on bone health: a literature review // Front. Endocrinol. (Lausanne). 2022. Vol. 13. Article ID 899375. DOI: https://doi.org/10.3389/fendo.2022.899375
Translation of the main text (without abstract, originally published in Russian):
"
The number of people who consume only plant and dairy foods with the rejection of meat is increasing every year around the world. People who exclude slaughter foods are called vegetarians, and those who exclude all animal products are called vegans [1]. Thus, while the diet of vegans is exclusively plant-based (not counting mushrooms), [lacto-ovo-]vegetarians can also consume both dairy products and eggs. The prevalence of vegetarianism is 36% in India and 5% in the United Kingdom [2]. A survey conducted by the Zoom Market research agency [?] in 2019 showed that in Russia, among 4080 respondents, 2% were vegetarians, mainly in St. Petersburg and Kaliningrad [3]. The motives that encourage people to choose vegetarianism are diverse: moral and ethical, environmental, economic, medical, religious [4]. It should be noted that in a number of diseases, limiting the consumption of meat and offal is medically indicated [5, 6]. However, adherents of such a diet [vegetarian diet] may be at a greater risk of developing osteopenia and osteoporosis [7].Osteoporosis is a systemic skeletal disease characterized by low bone mineral density (BMD) and impaired microarchitecture, which increase the risk of fractures [8, 9]. Depending on age and sex, it can be distinguised between idiopathic juvenile osteoporosis, idiopathic adult osteoporosis, postmenopausal osteoporosis, and senile osteoporosis. Osteoporosis can also develop in associatioon with a lack of physical activity, with endocrine, hematological, oncological, nephrological, gastroenterological, neurological, rheumatological diseases, or as due to iatrogenic effects, etc. [10].
Despite ample evidence of the beneficial effects of limiting the consumption of animal products and refined plant foods [11-13], the impact of dietary habits, including vegetarianism, on BMD is not fully understood. In this regard, BMD studies with vegans and vegetarians are very relevant today.
The aim of the [present] work was to study BMD in vegetarians, vegans, and people with a mixed diet [omnivores].
Material and methods
Research objects [:]
An observational cross-sectional study was carried out at the clinic of the Federal State Budgetary Institution "Federal Research Center for Nutrition and Biotechnology". 103 individuals, aged 18 to 77 years, with different diets were examined on an outpatient basis (Table 1).
Older than 18 years and self-identified as vegan, vegetarian, or non-vegan/non-vegetarian for the mixed diet group. Individuals with a mixed diet did not have any dietary restrictions and did not follow a strict diet for any reason.
The criterion for exclusion from the study was the presence of serious physical or mental illness.
Voluntary written informed consent was obtained from all study participants. The study was approved by the local ethics committee of the Federal State Budgetary Institution "Federal Research Center for Nutrition and Biotechnology" (protocol of the ethics committee number 6 of December 22, 2017).
All participants were divided into 4 samples.
The criterion for exclusion from the study was the presence of serious physical or mental illness.
Voluntary written informed consent was obtained from all study participants. The study was approved by the local ethics committee of the Federal State Budgetary Institution "Federal Research Center for Nutrition and Biotechnology" (protocol of the ethics committee number 6 of December 22, 2017).
All participants were divided into 4 samples.
Sample 1 included all the subjects (n=103, see Table 1).
Sample 2 were people under 50 years of age (n=84; 35 vegans, 26 vegetarians, 23 people with a mixed diet).
Sample 3 consisted of 26 vegans, 34 vegetarians and 26 people with a mixed diet who did not take additional vitamin D on a regular basis (n=86). This technique was used for the reason that vitamin D is a powerful stimulator of bone formation, including due to the fact that it significantly enhances the absorption of calcium in the small intestine, the bioavailability of which is usually significantly reduced in the case of plant-based diets [from plant foods] [14], and the deficiency of this vitamin is extremely common [15].
Sample 4 included 68 subjects (24 vegans, 23 vegetarians, 21 mixed dieters) who met both the above criteria (i.e., over 50 years of age and regular intake of vitamin D).
This strategy was chosen in order to comprehensively assess the state of bone tissue in vegetarians and vegans, i.e., the exclusion of the influence of vitamin D (sample 3) taking into account age (sample 4) and the characteristics of vegetarianism as a lifestyle (samples 1 and 2). A person was considered to be supplementing vitamin D if they declared their regular [supplementary] intake at any dose over the past 3 years.
Research methods[:]
To assess BMD, X-ray dual-energy absorptiometry (DXA) was used [8, 9]. The essence of the method lies in the fact that two X-ray beams of different intensity pass through the skeletal structures. Depending on the amount of radiation passing through the bones, that is not at the same time absorbed by adipose or muscle tissue, it is possible to calculate the content of mineral mass in the bones.
Strictly speaking, DXA does not measure BMD directly. The result of the study is an indicator of "two-dimensional bone density" expressed in g/cm2, and therefore the diagnosis of osteopenia and osteoporosis is based not on the absolute values of BMD, as in the case of computed tomography [CT scan] [16], but on the ratio of the obtained values to two reference values: [either] a reference population [specified] by age and ethnicity (Z-test) or a reference population of young people (T-test) [10, 17]. According to Russian clinical guidelines for the diagnosis of osteoporosis [10], the T-test should be used when working with women of peri- and postmenopausal age and men over 50 years old. For young adults and children, the Z-test should be used. In the present work, absolute indicators of "two-dimensional BMD", hereafter simply referred to as BMD, were determined for all examined patients, and T- and Z-criteria were calculated as standard deviation (SD) from the average expected BMD in [either] young people [i.e., T-score] or [compared to] the reference value based on age [i.e., Z-score]. The World Health Organization defines osteopenia as a T-score value of [the result being in the range of] -1.0 to -2.5, while osteoporosis is [defined as being] below -2.5 [9].
In this study, a Stratos X-ray osteodensitometer (DMS, France) was used, measurements were taken in the lumbar spine (LI-LIV) and in the neck of the left femur.
Statistical analysis of the results was carried out using the IBM SPSS version 23.0 (IBM, USA). The evaluation of the parameters for the normal distribution was performed using the Kolmogorov-Smirnov test. The distribution of all indicators in all 12 samples (4 samples, each with 3 populations) was normal. So the data are presented as means (M) and standard deviations (δ). Pairwise differences were assessed using Student's t-test. The Bonferroni correction was used to reduce the risk of false positive results when looking for multiple between-group differences. The calculated T- and Z-criteria were compared with the threshold values for osteopenia and osteoporosis. Thus, the proportions of individuals with these conditions [osteopenia and osteoporosis] were calculated. Comparison of the proportions of patients with deviations from the reference value in densitometric parameters between groups was carried out using Fisher's exact test.
Strictly speaking, DXA does not measure BMD directly. The result of the study is an indicator of "two-dimensional bone density" expressed in g/cm2, and therefore the diagnosis of osteopenia and osteoporosis is based not on the absolute values of BMD, as in the case of computed tomography [CT scan] [16], but on the ratio of the obtained values to two reference values: [either] a reference population [specified] by age and ethnicity (Z-test) or a reference population of young people (T-test) [10, 17]. According to Russian clinical guidelines for the diagnosis of osteoporosis [10], the T-test should be used when working with women of peri- and postmenopausal age and men over 50 years old. For young adults and children, the Z-test should be used. In the present work, absolute indicators of "two-dimensional BMD", hereafter simply referred to as BMD, were determined for all examined patients, and T- and Z-criteria were calculated as standard deviation (SD) from the average expected BMD in [either] young people [i.e., T-score] or [compared to] the reference value based on age [i.e., Z-score]. The World Health Organization defines osteopenia as a T-score value of [the result being in the range of] -1.0 to -2.5, while osteoporosis is [defined as being] below -2.5 [9].
In this study, a Stratos X-ray osteodensitometer (DMS, France) was used, measurements were taken in the lumbar spine (LI-LIV) and in the neck of the left femur.
Statistical analysis of the results was carried out using the IBM SPSS version 23.0 (IBM, USA). The evaluation of the parameters for the normal distribution was performed using the Kolmogorov-Smirnov test. The distribution of all indicators in all 12 samples (4 samples, each with 3 populations) was normal. So the data are presented as means (M) and standard deviations (δ). Pairwise differences were assessed using Student's t-test. The Bonferroni correction was used to reduce the risk of false positive results when looking for multiple between-group differences. The calculated T- and Z-criteria were compared with the threshold values for osteopenia and osteoporosis. Thus, the proportions of individuals with these conditions [osteopenia and osteoporosis] were calculated. Comparison of the proportions of patients with deviations from the reference value in densitometric parameters between groups was carried out using Fisher's exact test.
Results
[Sample 1, i.e., all participants]Overall, osteopenia was diagnosed more frequently in the lumbar spine than in the femoral neck. The frequency of detection of osteopenia in the participants of the different groups in the total sample is shown in Fig. 1.


[They mean prevalence, not incidence.]
Among vegetarians, osteoporosis in the spine occurred in 7 (18.4%) cases, while osteoporosis was not diagnosed in vegans. Among persons with a mixed diet, 2 (6.9%) participants had osteoporosis of the lumbar spine. Osteoporosis was not detected in the femoral neck in any of the examined patients. The T-score values in the lumbar spine in vegans were significantly higher than in vegetarians. T-score at the femoral neck did not differ between groups (Table 2). No significant differences in BMD were found in all samples.
[Values are means +- standard deviation.]
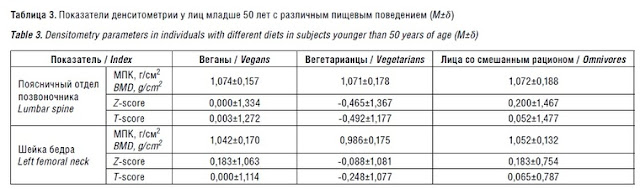
[Sample 2, i.e., those below 50 years old]
[Sample 2, i.e., those below 50 years old]
After the exclusion of persons older than 50 years, the same patterns were no longer observed. This is explained by the fact that, in sample 2, the largest number of persons from the group of vegetarians (n=12) was excluded compared to sample 1 [sample 1 = all participants], while among the vegans only 1 person was over 50 years old [and was thus excluded from sample 2] (Fig. 2, Table 3). ). Osteoporosis in sample 2 was diagnosed in 1 vegetarian, it was not found in the other [two] groups [i.e., vegans and omnivores].
[Values are means +- standard deviation.]
[Sample 3, i.e., those who did not seem to supplement vitamin D]
[Sample 3, i.e., those who did not seem to supplement vitamin D]
After exclusion of those participants who regularly took vitamin D supplements, differences between vegans and vegetarians reappeared: the prevalence of osteopenia in the lumbar spine among vegetarians was significantly higher than among vegans (Fig. 3), and T-scores in this zone [lumbar spine] was significantly lower [in vegetarians] than in vegans (Table 4). Diagnosable osteoporosis also reappeared among vegetarians and persons with a mixed diet: in 5 (14.7%) and 1 (3.8%) people, respectively.


[Values are means +- standard deviation.]
[Sample 4, i.e., those who supplemented vitamin D and were older than 50 years]
[Sample 4, i.e., those who supplemented vitamin D and were older than 50 years]
When considering individuals who met both ["]exclusion criteria["], no significant differences were found [between groups] (Table 5, Fig. 4).

[They mean prevalence, not incidence. I think, these are the people OLDER - not younger - than 50 years, who also did not seem to take vitamin D.]

[They mean prevalence, not incidence. I think, these are the people OLDER - not younger - than 50 years, who also did not seem to take vitamin D.]
Discussion
The study of the influence of vegetarianism on various aspects of human life and the most important functions of the body is currently becoming increasingly important. One of the most acute problems is the condition of bone tissue in vegans and vegetarians. This is due to the fact that it [bone health] is influenced by both nutrition and the lifestyle and age of the person. Thus, in persons older than 70 years, the rate of bone tissue resorption is approximately twice as high as in persons aged 60–69 years [18].Previous studies have found lower BMD in vegetarians than in omnivores [19]. In a paper by A. Welch et al., a 6% lower BMD was observed in vegetarian men, while women had normal BMD [20]. According to a meta-analysis from 2009, BMD was 4% lower in vegetarians and 6% lower in vegans than in those with a mixed diet [21]. However, in later works we see a different situation. In a study by L. Xie et al. (2019), BMD in young adult vegetarians did not differ from BMD in those with a mixed diet, but was significantly lower in vegans [22]. In a longitudinal study by T. L. Chuang et al., BMD reduction over 3 years among vegetarians aged 56–90 years did not differ from that in women with a mixed diet, although it was higher in vegetarians aged 40–55 years [23]. In 2018, E.Z. Movassagh et al. showed that the BMD of vegetarians may be higher than that of meat eaters [24]. Probably, a large number of studies on vegetarianism conducted in the past 20 years have played an important role in this, which has led to a significant increase in the quality of vegetarian and vegan diets [7]. At the same time, a recent study by J. Menzel et al., using quantitative ultrasonometry showed lower heel bone mineral density among German vegans compared to omnivores [25]; similar results were obtained in 2022 [26].
We did not find any such studies [on the topic of BMD in vegetarians/vegans] in Russia. In addition to the climatic and geographical features of the European territory of Russia, a huge contribution to the health of the population is also made by the features of economic development, the material well-being of the population, the national mentality, the availability of food products, and culinary preferences [this sound a little overenthusiastic]. All of the above predetermined the need to conduct a study of the state of bone tissue [bone health] in vegetarians and vegans in Russia.
It is known that physical activity interferes [which is a good thing] with bone resorption [27]. Vegetarians, especially women who are more active, may have lower bone resorption compared to mixed dieters [28, 29]. In a study by R. Wakolbinger-Habel et al. [30] found no differences in bone microarchitecture between vegans with regular strength training and omnivores, although quantitative indicators of BMD in non-exercising vegans were lower when compared with a similar group of omnivores.
The present study found no reduction in BMD in vegans compared to omnivores, other things being equal. The differences were mainly due to the large number of older persons among the surveyed vegetarians compared to the other [two] groups. At the same time, the fact of regular intake of vitamin D did not have the expected effect on the results of the study.
Study limitations
Limitations associated with the studied samples[:]
Small sample size, the gender and age heterogeneity were a significant limitation of the study. The greater number of peri- and postmenopausal women in the vegetarian group, we believe, significantly affected the results of the work.
The sample formed according to the criterion of "regular vitamin D intake" [presumably, they mean "sample 4"] was also highly heterogeneous in terms of the dose of vitamin D, its form (chole- or ergocalciferol) and the regimen of administration. Further splitting the sample according to the dose/intake regimen would not have been reasonable (as there was a very large variety of doses and [vitamin D] regimens among the participants) and [creating further, even smaller subgroups] would [have] critically reduce[d] the studied samples.
Study design limitations. The design of the study does not involve an in-depth analysis of the impact of a vegetarian or vegan diet on BMD, as it is not a longitudinal study. Being cross-sectional, the study offers purely empirical data on the prevalence of osteopenia or osteoporosis in 3 groups with different dietary patterns. In addition, it is difficult to assess the contribution of the diet itself to the risk of developing a particular pathology without analyzing the diet itself. When studying the impact of different ways of eating on bone health, it is important to assess protein intake, including essential amino acids [31], [as well as the intake of] calcium, magnesium, potassium, zinc, copper, manganese, silicon, vitamins D, A (and carotenoids), C, B6, B9 [folate], B12, ω-6- and ω-3-polyunsaturated fatty acids and some minor food components [32, 33].
Limitations related to the research methodology. DXA was the method for assessing the state of bone tissue [bone health]; [DXA is] the "gold standard" for diagnosing osteoporosis today. However, DXA is not the most accurate research method. Firstly, with DXA, it is not possible to assess the state of cancellous bone, from the defeat of which osteoporosis usually manifests itself. Secondly, DXA does not [directly] assess BMD itself [16]. Finally, in tall people with low body weight, DXA often gives false positive results, since they often constitutionally have thinner cortical bone [9]. On the other hand, computed tomography [CT scan] that does not have these limitations incurs a much higher radiation exposure [radioactivity] and economic costs.
Caution should also be taken when interpreting the results of a single study of BMD, especially using the DXA method. Osteoporosis and osteopenia is a consequence of increased bone resorption, [which is] a pathological process. A single study of bone tissue [bone health], even if computed tomography is used, cannot show [an accurate] picture of the dynamics. Accordingly, a constitutionally low or insufficiently increased BMD at a young age will be regarded as osteopenia or osteoporosis if the BMD values in the case of computed tomography, or the T/Z-criterion in the case of DXA, are low. Of course, the risk of low-traumatic fractures depends only on the state of the bone tissue, and not on the causes of this condition, however, it is not possible to determine the patient's prognosis with a single densitometry [DXA scan or CT scan; i.e., bone mineral density assessment].
The sample formed according to the criterion of "regular vitamin D intake" [presumably, they mean "sample 4"] was also highly heterogeneous in terms of the dose of vitamin D, its form (chole- or ergocalciferol) and the regimen of administration. Further splitting the sample according to the dose/intake regimen would not have been reasonable (as there was a very large variety of doses and [vitamin D] regimens among the participants) and [creating further, even smaller subgroups] would [have] critically reduce[d] the studied samples.
Study design limitations. The design of the study does not involve an in-depth analysis of the impact of a vegetarian or vegan diet on BMD, as it is not a longitudinal study. Being cross-sectional, the study offers purely empirical data on the prevalence of osteopenia or osteoporosis in 3 groups with different dietary patterns. In addition, it is difficult to assess the contribution of the diet itself to the risk of developing a particular pathology without analyzing the diet itself. When studying the impact of different ways of eating on bone health, it is important to assess protein intake, including essential amino acids [31], [as well as the intake of] calcium, magnesium, potassium, zinc, copper, manganese, silicon, vitamins D, A (and carotenoids), C, B6, B9 [folate], B12, ω-6- and ω-3-polyunsaturated fatty acids and some minor food components [32, 33].
Limitations related to the research methodology. DXA was the method for assessing the state of bone tissue [bone health]; [DXA is] the "gold standard" for diagnosing osteoporosis today. However, DXA is not the most accurate research method. Firstly, with DXA, it is not possible to assess the state of cancellous bone, from the defeat of which osteoporosis usually manifests itself. Secondly, DXA does not [directly] assess BMD itself [16]. Finally, in tall people with low body weight, DXA often gives false positive results, since they often constitutionally have thinner cortical bone [9]. On the other hand, computed tomography [CT scan] that does not have these limitations incurs a much higher radiation exposure [radioactivity] and economic costs.
Caution should also be taken when interpreting the results of a single study of BMD, especially using the DXA method. Osteoporosis and osteopenia is a consequence of increased bone resorption, [which is] a pathological process. A single study of bone tissue [bone health], even if computed tomography is used, cannot show [an accurate] picture of the dynamics. Accordingly, a constitutionally low or insufficiently increased BMD at a young age will be regarded as osteopenia or osteoporosis if the BMD values in the case of computed tomography, or the T/Z-criterion in the case of DXA, are low. Of course, the risk of low-traumatic fractures depends only on the state of the bone tissue, and not on the causes of this condition, however, it is not possible to determine the patient's prognosis with a single densitometry [DXA scan or CT scan; i.e., bone mineral density assessment].
Conclusion
The present study found no reduction in BMD in vegans compared to omnivores, other things being equal. The higher incidence of osteopenia and osteoporosis among vegetarians [in this study] is primarily due to the presence of more people over 50 years of age in this group.Moreover, when comparing subjects who did not take vitamin D, mean BMD values in both the femoral neck and lumbar vertebrae were slightly higher among vegans, although there were no statistically significant differences [between groups].
The data obtained do not allow us to make an unambiguous conclusion about the higher or lower prevalence of demineralizing disease among vegans and vegetarians compared to people with a mixed diet [omnivores].
Funding
The research work on the preparation of the manuscript was carried out at the expense of a subsidy for the fulfillment of the state task under the Program of Fundamental Scientific Research of the State Academies of Sciences for 2022-2024. (reference number: FGMF-2022-0005).Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
Concept and design of the study - Galchenko A.V., Gapparova K.M., Revyakina V.A.
Data collection and statistical analyses, writing of the article - Galchenko A.V., Sidorova E.I., Sherstneva A.A.
Editing, approval of the final version of the article, responsibility for the integrity of all parts of the article - all authors
References
1. Agnoli C., Baroni L., Bertini I., Ciappellano S., Fabbri A., Papa M. et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition // Nutr. Metab. Cardiovasc. Dis. 2017. Vol. 27, N 12. P. 1037-1052. DOI: https://doi.org/10.1016/j.numecd.2017.10.0202. Chai Z.F., Gan W.Y., Chin Y.S., Ching Y.K., Appukutty M. Factors associated with anemia among female adult vegetarians in Malaysia // Nutr. Res. Pract. 2019. Vol. 13, N 1. P. 23. DOI: https://doi.org/10.4162/nrp.2019.13.1.23
3. Исследовательское агентство Zoom Market. URL: https://www.mazm.ru/article/a-2122.php (дата обращения: 28.03.2019).
4. Elorinne A., Alfthan G., Erlund I. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians // PLoS One. 2016. Vol. 11, N 2. Article ID e0148235. DOI: https://doi.org/10.1371/journal.pone.0148235
5. Kahleova H., Levin S., Barnard N. Cardio-metabolic benefits of plant-based diets // Nutrients. 2017. Vol. 9, N 8. P. 848. DOI: https://doi.org/10.3390/nu9080848
6. Zhubi-Bakija F., Bajraktari G., Bytyçi I., Mikhailidis D.P., Henein M.Y., Latkovskis G. et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the International Lipid Expert Panel (ILEP) // Am. J. Clin. Nutr. 2020. Vol. 40, N 1. P. 255-276. DOI: https://doi.org/10.1016/j.clnu.2020.05.017
7. Galchenko A., Gapparova K., Sidorova E. The influence of vegetarian and vegan diets on the state of bone mineral density in humans // Crit. Rev. Food. Sci. Nutr. 2023. Vol. 63, N 7. Р. 845-861. DOI: https://doi.org/10.1080/10408398.2021.1996330
8. Lane J.M., Russell L., Khan S.N. Osteoporosis // Clin. Orthop. Relat. Res. 2000. Vol. 372. P. 139-150. DOI: https://doi.org/10.1097/00003086-200003000-00016
9. Karaguzel G., Holick M.F. Diagnosis and treatment of osteopenia // Rev. Endocr. Metab. Disord. 2010. Vol. 11, N 4. P. 237-251. DOI: https://doi.org/10.1007/s11154-010
10. Мельниченко Г.А., Белая Ж.Е., Рожинская Л.Я., Торопцова Н.В., Алексеева Л.И., Бирюкова Е.В. и др. Федеральные клинические рекомендации по диагностике, лечению и профилактике остеопороза // Проблемы эндокринологии. 2017. Т. 63, № 6. С. 392-426. DOI: https://doi.org/10.14341/probl2017636392-426
11. Hu J., Li Y., Wang Z., Li X., Hou T., Ning Z. et al. Association of plant-based dietary patterns with the risk of osteoporosis in community-dwelling adults over 60 years: a cross-sectional study // Osteoporos. Int. 2023. Vol. 34, N 5. Р. 915-923. DOI: https://doi.org/10.1007/s00198-023-06700-2
12. Ghadiri M., Cheshmazar E., Shateri Z., Gerami S., Nouri M., Gargari B.P. Healthy plant-based diet index as a determinant of bone mineral density in osteoporotic postmenopausal women: a case-control study // Front. Nutr. 2023. Vol. 9. Article ID 1083685. DOI: https://doi.org/10.3389/fnut.2022.1083685
13. Zheng Y., Wang J., Wang Y., Xu K., Chen X. The Hidden dangers of plant-based diets affecting bone health: a cross-sectional study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005-2018 // Nutrients. 2023. Vol. 15, N 7. Р. 1794. DOI: https://doi.org/10.3390/nu15071794
14. Galchenko A.V., Ranjit R. Calcium status among vegetarians and vegans // Российской научно-практической конференции с международным участием "Фундаментальные основы технологического развития сельского хозяйства" : сборник трудов. Оренбург: Федеральное государственное бюджетное научное учреждение "Федеральный научный центр биологических систем и агротехнологий Российской академии наук", 2019. С. 214-217.
15. Galchenko A.V., Ranjit R. Vitamin D and its status in vegetarians and vegans // Вопросы биологической, медицинской и фармацевтической химии. 2021. Т. 24, № 11. С. 20-27. DOI: https://doi.org/10.29296/25877313-2021-11-04
16. Шкарабуров А.С., Колпинский Г.И., Захаров И.С., Шкарабуров С.П., Мозес В.Г. Использование лучевых методов в диагностике постменопаузального остеопороза // Фундаментальная и клиническая медицина. 2017. Т. 2, № 2. С. 70-76. DOI: https://doi.org/10.23946/2500-0764-2017-2-2-70-76
17. Oral A., Esmaeilzadeh S., Yalıman A., Sindel D., Kürsüz Köseoğlu P., Aydın T. The ability of calcaneal and multisite quantitative ultrasound variables in the identification of osteoporosis in women and men // Turk. J. Phys. Med. Rehabil. 2019. Vol. 65, N 3. P. 203-215. DOI: https://doi.org/10.5606/tftrd.2019.1894
18. Ho-Pham L.T., Vu B.Q., Lai T.Q., Nguyen N.D., Nguyen T.V. Vegetarianism, bone loss, fracture and vitamin D: a longitudinal study in Asian vegans and non-vegans // Eur. J. Clin. Nutr. 2012. Vol. 66, N 1. P. 75-82. DOI: https://doi.org/10.1038/ejcn.2011.131
19. Craig W.J. Health effects of vegan diets // Am. J. Clin. Nutr. 2009. Vol. 89, N 5. P. 1627S-1633S. DOI: https://doi.org/10.3945/ajcn.2009.26736N
20. Welch A., Bingham S., Camus J., Dalzell N., Reeve J., Day N., Khaw K.T. Calcaneum broadband ultrasound attenuation relates to vegetarian and omnivorous diets differently in men and women: an observation from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study // Osteoporos. Int. 2005. Vol. 16, N 6. P. 590-596. DOI: https://doi.org/10.1007/s00198-004-1721-3
21. Ho-Pham L.T., Nguyen N.D., Nguyen T.V. Effect of vegetarian diets on bone mineral density: a Bayesian meta-analysis // Am. J. Clin. Nutr. 2009. Vol. 90, N 4. P. 943-950. DOI: https://doi.org/10.3945/ajcn.2009.27521
22. Xie L., Wang B., Cui X., Tang Q., Cai W., Shen X. Young adult vegetarians in Shanghai have comparable bone health to omnivores despite lower serum 25(OH) vitamin D in vegans: a cross-sectional study // Asia Pac. J. Clin. Nutr. 2019. Vol. 28, N 2. P. 383-288. DOI: https://doi.org/10.6133/apjcn.201906_28(2).0021
23. Chuang T.L., Koo M., Chuang M.H., Lin C.H., Huang C.H., Wang Y.F. Changes in bone mineral density and trabecular bone score over time between vegetarian and non-vegetarian middle-aged and older women: a three-year retrospective medical record review // Int. J. Environ. Res. Public Health. 2022. Vol. 19, N 4. Р. 2445. DOI: https://doi.org/10.3390/ijerph19042445
24. Movassagh E.Z., Baxter-Jones A.D.G., Kontulainen S., Whiting S., Szafron M., Vatanparast H. Vegetarian-style dietary pattern during adolescence has long-term positive impact on bone from adolescence to young adulthood: a longitudinal study // Nutr. J. 2018. Vol. 17, N 1. P. 36. DOI: https://doi.org/10.1186/s12937-018-0324-3
25. Menzel J., Abraham K., Stangl G.I., Ueland P.M., Obeid R., Schulze M.B. et al. Vegan diet and bone health - results from the cross-sectional RBVD study // Nutrients. 2021. Vol. 13, N 2. Р. 685. DOI: https://doi.org/10.3390/nu13020685
26. Penczynski K.J., Remer T., Menzel J., Abraham K., Weikert C. Urinary potential renal acid load (uPRAL) among vegans versus omnivores and its association with bone health in the cross-sectional risks and benefits of a vegan diet study // Nutrients. 2022. Vol. 14, N 21. Р. 4468. DOI: https://doi.org/10.3390/nu14214468
27. Aguado E., Pascaretti-Grizon F., Goyenvalle E., Audran M., Chappard D. Bone mass and bone quality are altered by hypoactivity in the chicken // PLoS One. 2015. Vol. 10, N 1. Article ID e0116763. DOI: https://doi.org/10.1371/journal.pone.0116763
28. Bedford J.L., Barr S.I. Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more "health conscious" // Int. J. Behav. Nutr. Phys. Act. 2005. Vol. 2, N 1. P. 4. DOI: https://doi.org/10.1186/1479-5868-2-4
29. Tong T.Y.N., Appleby P.N., Armstrong M.E.G., Fensom G.K., Knuppel A., Papier K. et al. Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study // BMC Med. 2020. Vol. 18, N 1. P. 353. DOI: https://doi.org/10.1186/s12916-020-01815-3
30. Wakolbinger-Habel R., Reinweber M., König J., Pokan R., König D., Pietschmann P. et al. Self-reported resistance training is associated with better HR-pQCT-derived bone microarchitecture in vegan people // J. Clin. Endocrinol. Metab. 2022. Vol. 107, N 10. Р. 2900-2911. DOI: https://doi.org/10.1210/clinem/dgac445
31. Гальченко А.В., Морозова Л.Д., Залетова Т.С. Оценка потребности в белке и аминокислотах, исходя из биосинтетических потребностей и показателей азотистого баланса // Вопросы диетологии. 2017. Т. 7, № 2. С. 64-68. DOI: https://doi.org/10.20953/2224-5448-2017-2-64-68
32. Гальченко А.В. Влияние факторов образа жизни на метаболизм костной ткани и риск развития остеопороза // Профилактическая медицина. 2022. Т. 25, № 6. С. 96-107. DOI: https://doi.org/10.17116/profmed20222506196
33. Falchetti A., Cavati G., Valenti R., Mingiano C., Cosso R., Gennari L. et al. The effects of vegetarian diets on bone health: a literature review // Front. Endocrinol. (Lausanne). 2022. Vol. 13. Article ID 899375. DOI: https://doi.org/10.3389/fendo.2022.899375
"